Formwork
The most affordable eQMS software for agile, lean, founder-lead companies.
For EU MDR and FDA compliance. For Software and Hardware devices.
Pricing: Free, 99€, or 499€ per month. Cancel any time, no commitment.
On the market since over 5 years.
Passed EU MDR, FDA and Health Canada audits.
Thousands of companies.








Watch The Quick Walkthrough

We're collaborating with BerlinCert, a Notified Body focused on Software as a Medical Device (SaMD), on data formats for audit submissions. Learn more here.
What is it?
Formwork is a cloud-based, GDPR-compliant eQMS software for medical device startups. Document review, versioning and archiving. Training and CAPA management. Technical documentation for your medical device. Risk management and usability data tracking. All you need to pass your audits, in one place.
OK, what's the catch?
There is no catch. We’re just a bunch of people who are tired of shady practices in the eQMS software space and medical compliance in general. We built Formwork to help startups like yours get their medical devices certified while being transparent and fair. We’re not here to lock you in or charge you a fortune for adding more than two users.
Take a closer look
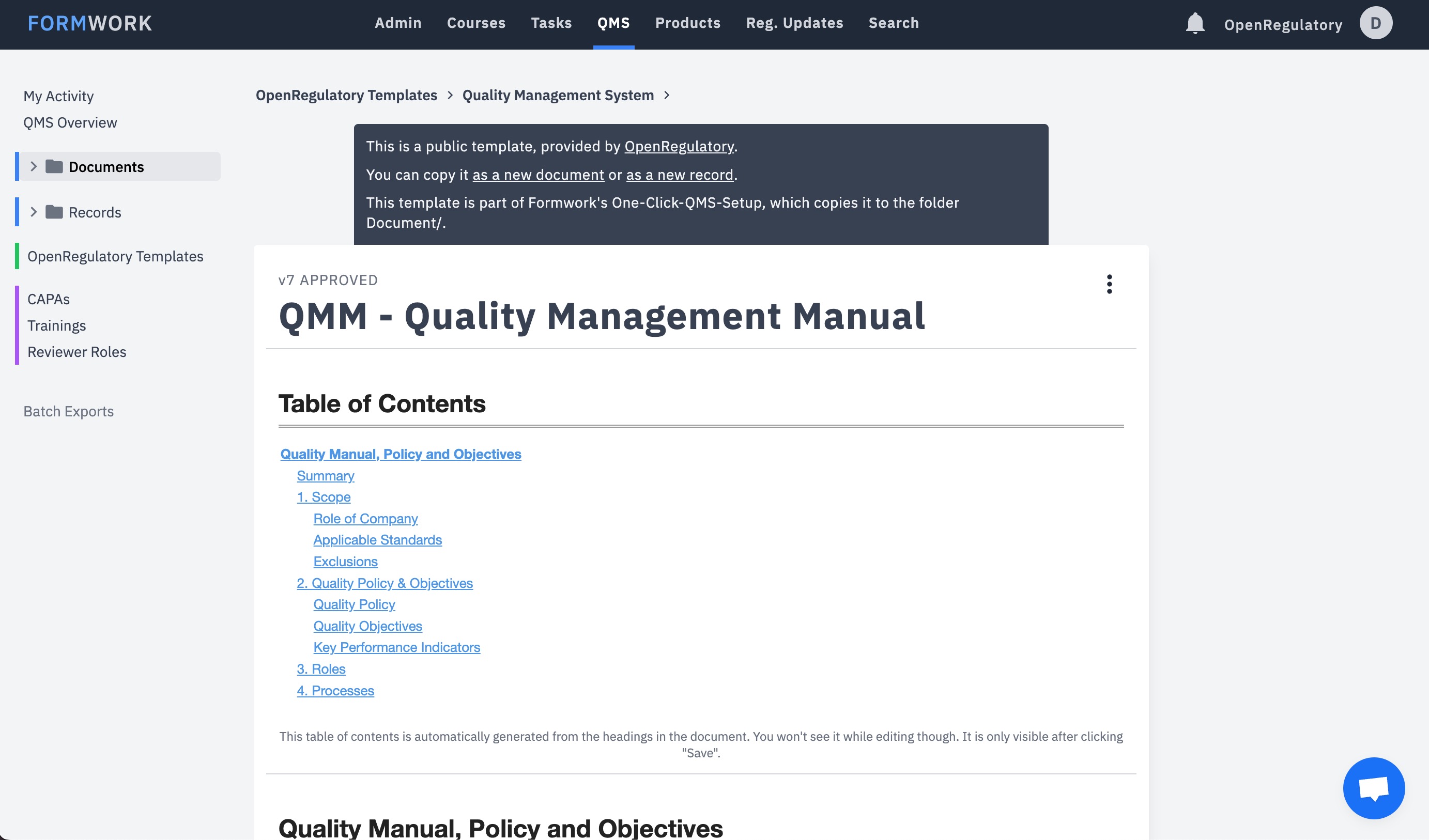
Use our templates and text editor to create your documents. Review and sign them with our built-in workflow.
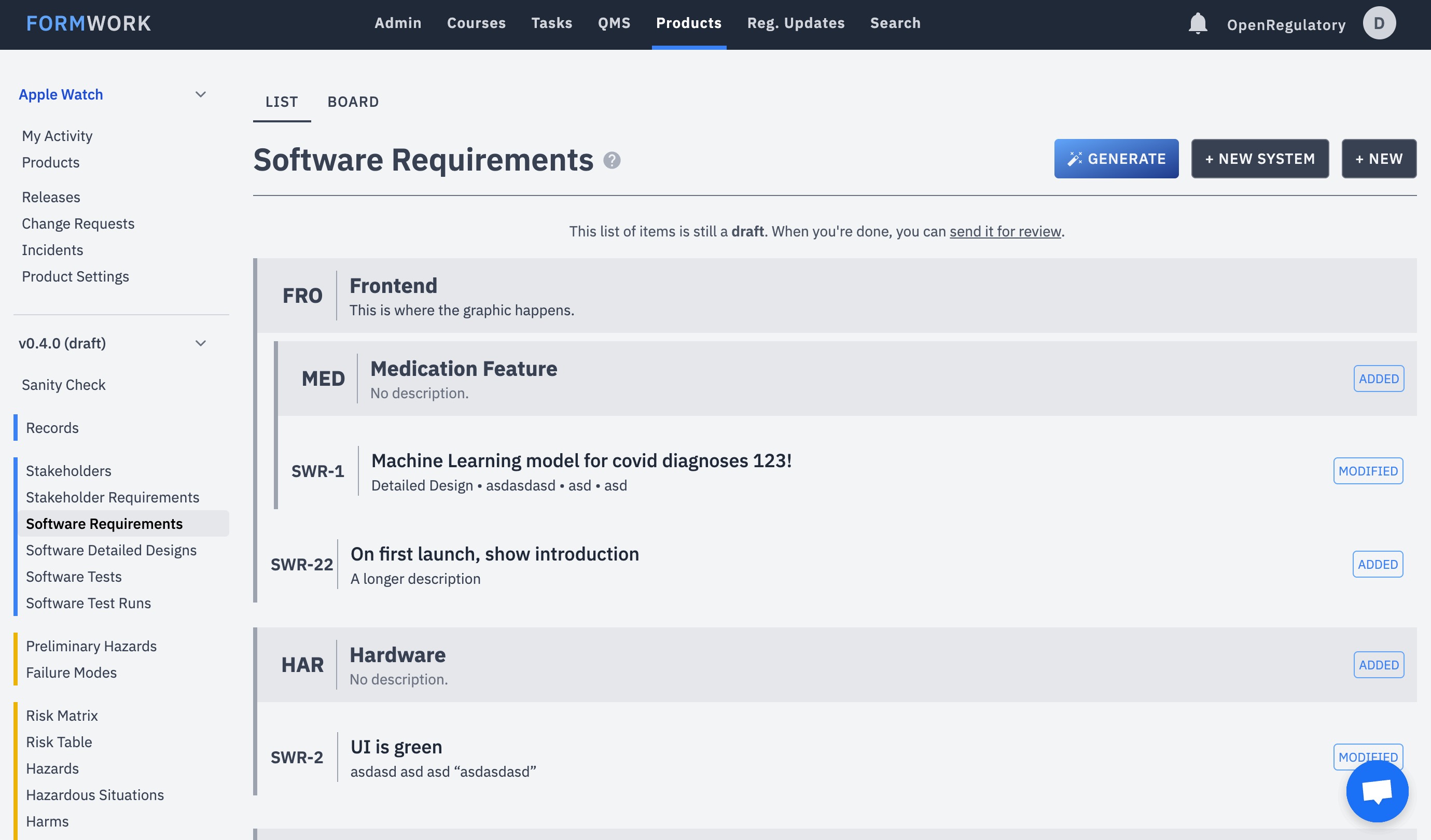
User-friendly way to manage the technical documentation for your medical device. Software or hardware, we support both!
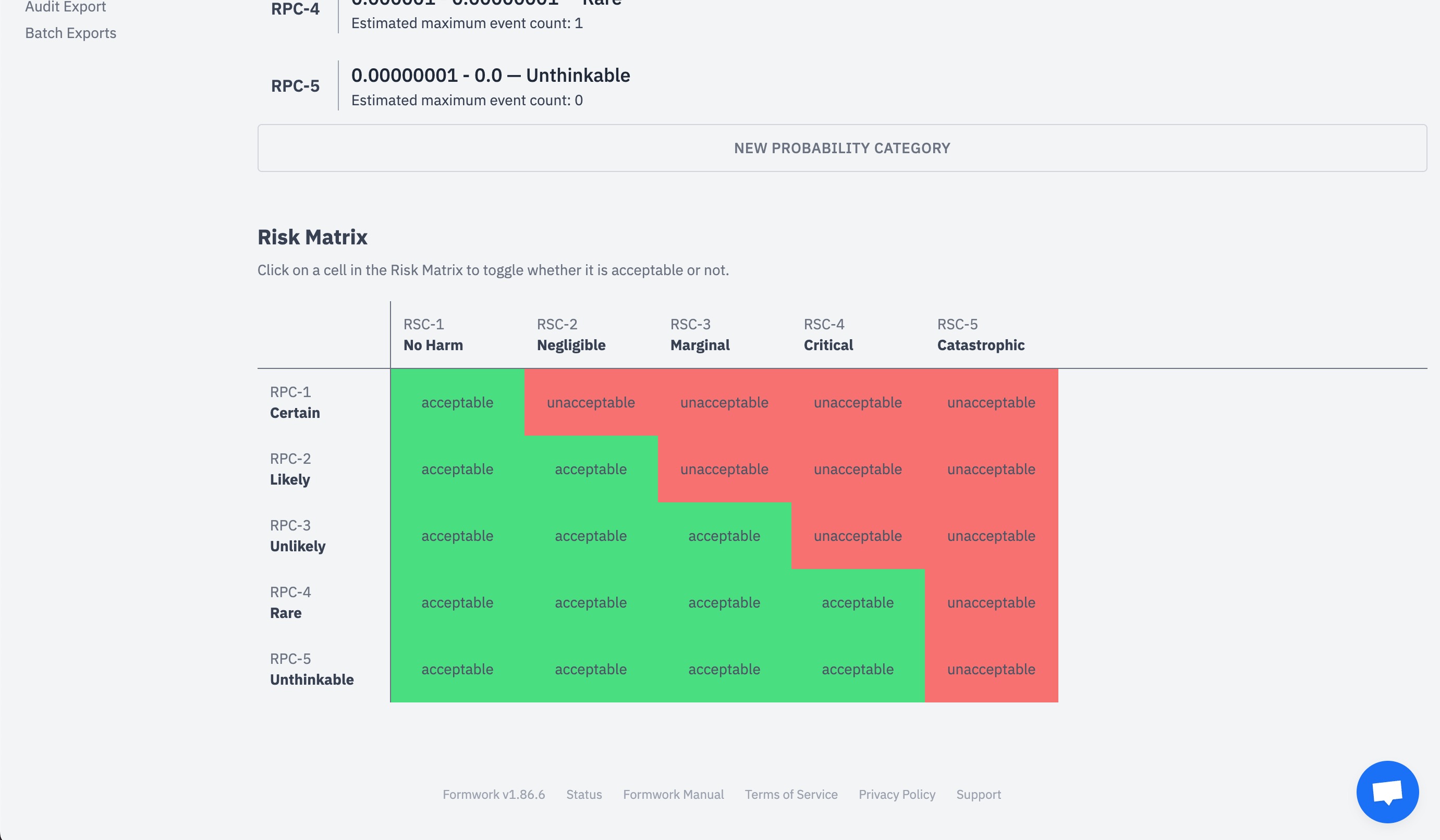
Risk management with great visualizations.
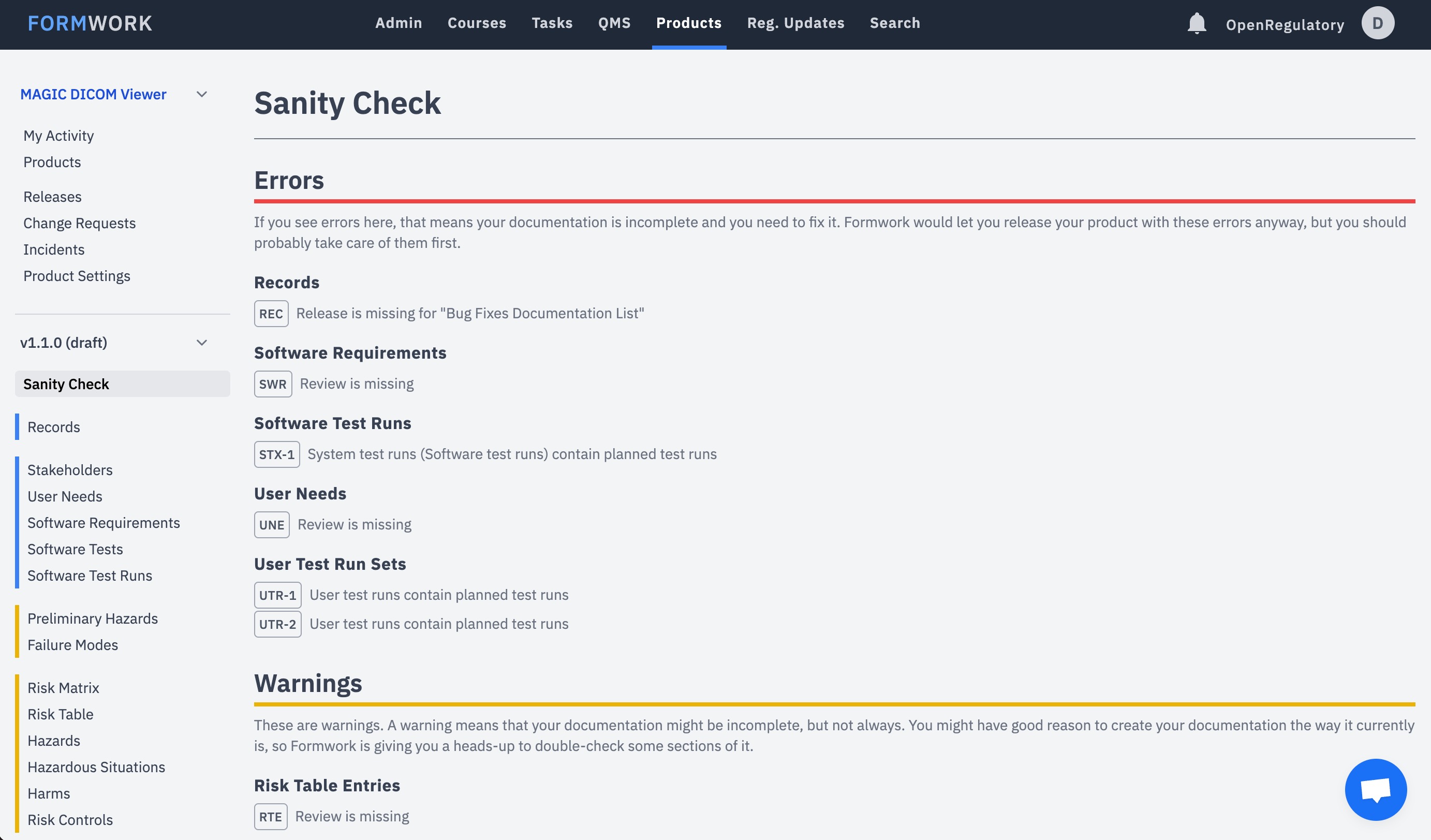
You get instant feedback regarding the completeness of your technical documentation.
Testimonials
Startups pass audits with Formwork
Our customers have passed EU MDR, FDA and Health Canada audits.
Click on each testimonial to read the full story.
"OpenRegulatory's Formwork offers distinct advantages to startups embarking on their regulatory compliance journey."

"With Formwork, we successfully set up our quality management system and also used it to create the technical documentation for our class IIa medical device. We were particularly helped by the numerous explanatory videos and the easily usable templates."

"If I could do it all again, I would choose Formwork."

"Formwork made it really easy for us to comply with the US FDA requirements. All our QMS SOPs and required records are now completely managed within the same simple and intuitive system. Huge win!"

"As a startup that initially managed our QMS entirely in Google Drive, we realized how difficult and unsustainable that approach was. After researching various eQMS options, we were beyond grateful to discover OpenRegulatory’s Formwork. There’s truly nothing else like it."

"Hang on, this regulatory stuff is actually quite straightforward when someone who actually DEVELOPS stuff, is explaining it to you, because they don't make up bullshit terms to confuse you."

Pricing & Features
Yes, these are flat prices per month, up to 20x cheaper than our competitors.
Check out our pricing page for a more detailed breakdown of pricing and features.
Formwork is 6x - 40x cheaper than our competitors.
Community Edition
Free
Free forever!
Free for everyone and perfect for you to get started. Better than Google Drive, Jira and Sharepoint. Access to all features, with some usage limitations.
- 5 user seats Perfect for up to five dudes or dudesses working in a garage.
- QMS Document Management For ISO 13485 (limited) Store rich-text QMS documents and records, with usage limitations.
- Technical Documentation Management (limited) Manage your software requirements (IEC 62304), risks (ISO 14971) and usability (IEC 62366).
- FDA 21 CFR Part 11 Compliance Sign your documents with FDA 21 CFR Part 11 compliant e-signatures.
- Advanced AI Features Generate your QMS with our AI which pre-fills your documents for you.
- Customer & Audit Support Reach out if you need help with technical problems, especially if you're in an audit.
- Release Your Product Finalize your documentation for your audit by releasing your product documentation.
- ISO 13485 Validation Report Receive an ISO 13485 - compliant validation report of Formwork.
Formwork Starter
99€ / month
+ VAT, billed monthly
Almost all features of the 499€ tier. Perfect for preparing everything for your certification. No usage limitations except product releases. Includes AI features.
- 5 user seats Perfect for up to five dudes or dudesses working in a garage.
- QMS Document Management For ISO 13485 (unlimited) Store rich-text QMS documents and records.
- Technical Documentation Management (unlimited) Manage your software requirements (IEC 62304), risks (ISO 14971) and usability (IEC 62366).
- FDA 21 CFR Part 11 Compliance Sign your documents with FDA 21 CFR Part 11 compliant e-signatures.
- Advanced AI Features Generate your QMS with our AI which pre-fills your documents for you.
- Customer & Audit Support (limited) Reach out if you need help with technical problems, especially if you're in an audit.
- Release Your Product Finalize your documentation for your audit by releasing your product documentation.
- ISO 13485 Validation Report Receive an ISO 13485 - compliant validation report of Formwork.
Formwork QMS + Techdoc
499€ / month
+ VAT, billed monthly
Everything you need for your medical device certification - set up your QMS with AI and document your EU MDR and/or FDA medical devices. No limitations.
- Unlimited user seats We won't delete your data if you downgrade.
- QMS Document Management For ISO 13485 (unlimited) Store rich-text QMS documents and records.
- Technical Documentation Management (unlimited) Manage your software requirements (IEC 62304), risks (ISO 14971) and usability (IEC 62366).
- FDA 21 CFR Part 11 Compliance Sign your documents with FDA 21 CFR Part 11 compliant e-signatures.
- Advanced AI Features Generate your QMS with our AI which pre-fills your documents for you.
- Customer & Audit Support Reach out if you need help with technical problems, especially if you're in an audit.
- Release Your Product Finalize your documentation for your audit by releasing your product documentation.
- ISO 13485 Validation Report Receive an ISO 13485 - compliant validation report of Formwork.
Frequently Asked Questions
No worries, it's quite simple, depending on your situation! If you're a startup on a budget, just get started with our free Community Edition. It's perfect for you to get started with your medical device documentation for free and has all features you need. In more technical terms, you get 5 user seats and can create e.g. up to 10 documents.
When you start growing and hit some of the usage limitations of the free tier, it's best to upgrade to Formwork Starter which only costs 99€ / month. With Starter, you can finish your entire medical device documentation for your first audit.
Once you're ready for your first audit, upgrade to QMS + Techdoc for 499€ / month. In contrast to Starter, you can finalize (release) your documentation with it, and you get unlimited user seats (vs. 5 on Starter).
TLDR: Get started with the free tier, purchase 99€ when you're creating more documentation and upgrade to 499€ just before your audit.
We did this in the past, but we no longer do this nowadays. We believe Formwork is really good value for its money.
We’ve got you covered! You can combine our templates with the magic of ChatGPT to create the first draft of your QMS in just a few minutes. Try it for yourself - check out the video further below!
Yes! Customers have passed MDR audits with Formwork. You can read their testimonials above. Will your company be next?
Yes! It depends what you mean by that though. If e.g. your auditor has technical questions about Formwork, we’ll be happy to answer those and provide you with explanations which have helped other customers pass audits successfully.
If you need an additional feature which you consider important for your audit, we’ll be happy to look into it. We’ve already built many such features for other customers, but we can’t guarantee we’ll build everything.
We don’t provide consulting though –
Yes! Just scroll down and click the "Schedule a demo" button.
No. As consultants, we’ve helped 150+ companies with their medical device compliance, and, when looking closely, we’ve never found a reasonable use case for an eQMS integration with Jira or any other development tool. Please read this article for more context on our decision.
Yes – we’ve got you covered. Formwork fully supports the requirements management, test case management, risk and usability management for hardware devices.
Formwork users include manufacturers targeting EU MDR compliance as well as some (much less) companies going for FDA compliance. Looking at the EU MDR companies, they either go for class I, IIa or IIb approval. Class I is the most common. To our knowledge, we haven’t seen a company use it for a class III device… yet!
Yes. Multiple of our customers have passed FDA audits with Formwork.
Yes. At least one of our customers has passed a Health Canada audit with Formwork.
Yes! You can export everything as PDF, Markdown or Excel files.
Yes, Formwork is cloud-based. The data is located in France, and Germany for redundancy. We use French and German hosting providers. We don’t use US-based cloud providers like Amazon, Google and Microsoft, due to GDPR concerns.
No, unfortunately not. We’re a small team, and offering a self-hosted option while also providing technical support at reasonable prices didn’t seem feasible to us. We only offer Formwork as cloud-hosted tool.
We save all our data redundantly on multiple servers: Our SQL servers are mirrored and our object storage is, too.
No. That’s because the free Community Edition has usage limitations, e.g. storing up to 10 documents. Pretty much any medical device QMS documentation will contain more than 10 documents. So the free tier is perfect to get you started, but you’ll need to upgrade to e.g. Formwork Starter (99€ / month) to complete the first draft of your medical device documentation.
Not too hard, but definitely some work. You can export your data any time as PDF, Markdown or Excel files – you can start it yourself and it takes a few minutes. You’ll have to put in some work actually integrating this data into your new system, of course. Contrast this with other eQMS providers which don’t even provide a data export like we do.
Other manufacturers have huge sales teams. We just have our website. Other manufacturers target large enterprise companies. We target startups and fast-moving companies. Other manufacturers put a lot of effort into keeping their software secret, because it actually sucks. We believe in transparency. This and many other reasons allow us to charge much lower prices. And we love startups.
Yes, you could do that (in theory)! However, 99% of people decide against this as you need to show Formwork to your auditors on an ongoing basis, and there's a lot of day-to-day compliance data you need to work on in Formwork (trainings etc.) - in any case, you'd need to move to another tool, and that other tool is likely going to cost you more time and money than Formwork.
If you’re a paying Formwork customer in the 499€ / month tier: Yes, we’ll provide you with a validation report which you can use for documentation of your QMS software validation. With this report, Formwork has been accepted by all auditors so far. Best of all, we provide you with this report free of charge while our competitors charge you huge additional fees!
Yes. There are two big reasons for this: OpenRegulatory has been profitable from the start, and the company is still 100% owned by Oliver, so there are no external (venture capital) investors which could force us to e.g. sell the company.
This is in huge contrast to most other eQMS software companies on the market: Qualio and Greenlight Guru are funded by venture capital investors, and Matrix Requirements recently got sold to a private equity fund. None of these things are bad by themselves, but it goes to show that founders in these situations tend to have (much) less control over their companies because they no longer own 100% of them.
Don't know where to start?
Formwork's AI has you covered.
Ready To Streamline Your Compliance?
Join thousands of fast-moving companies solving their medical device compliance with Formwork.
Start your free trial now, chat with us, or schedule a demo.
Got questions? Reach out any time, we're happy to help!