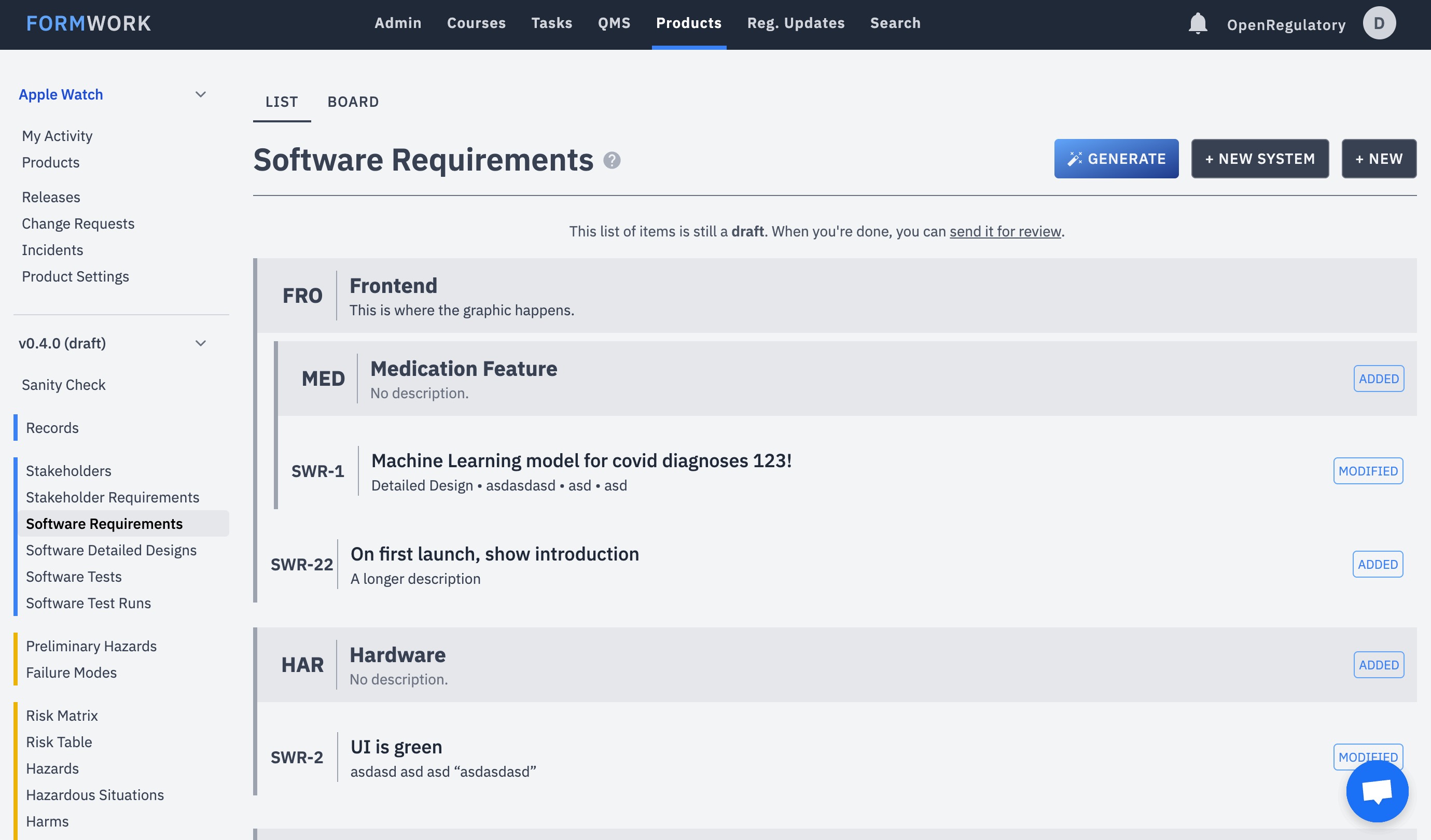
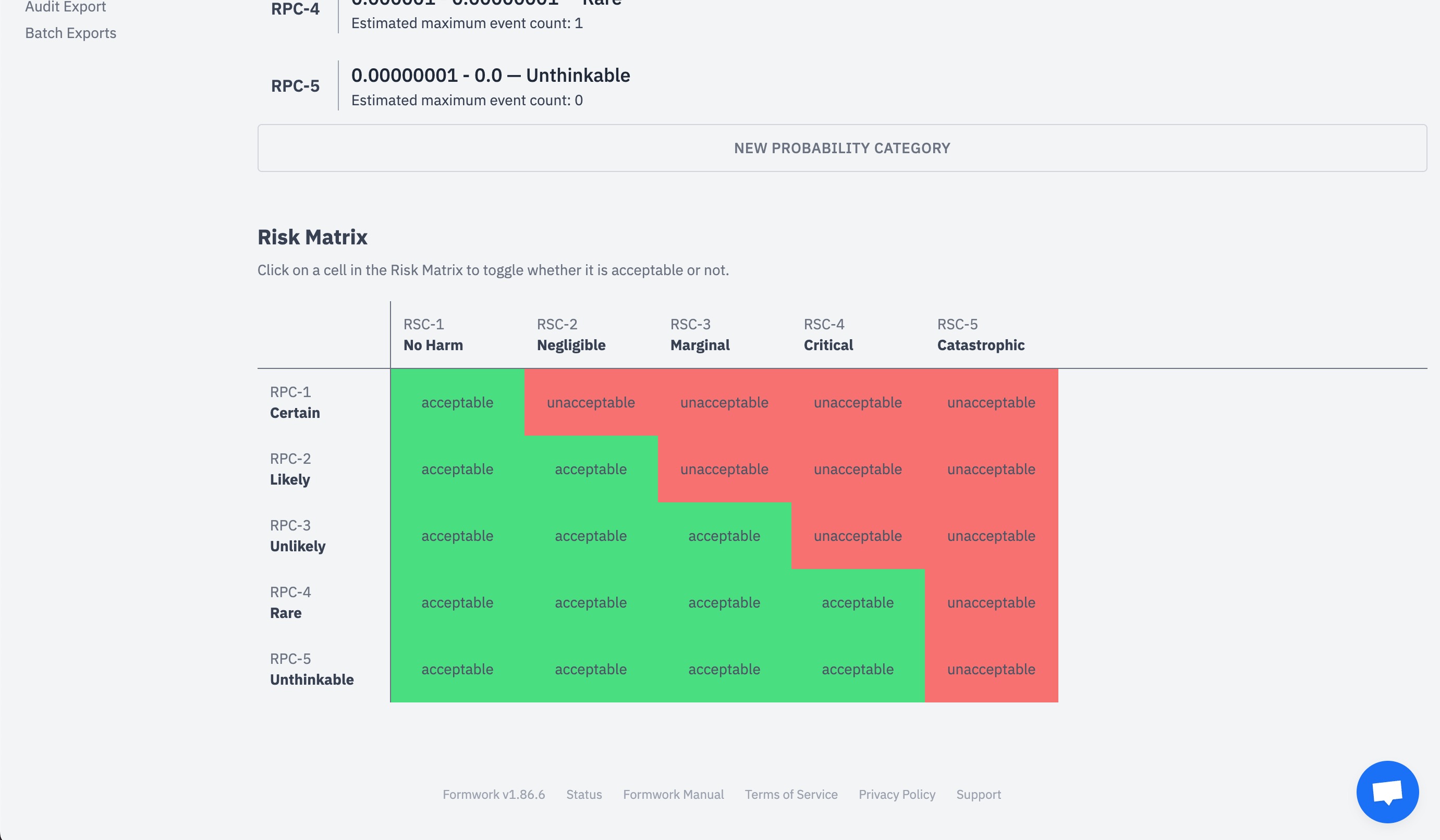
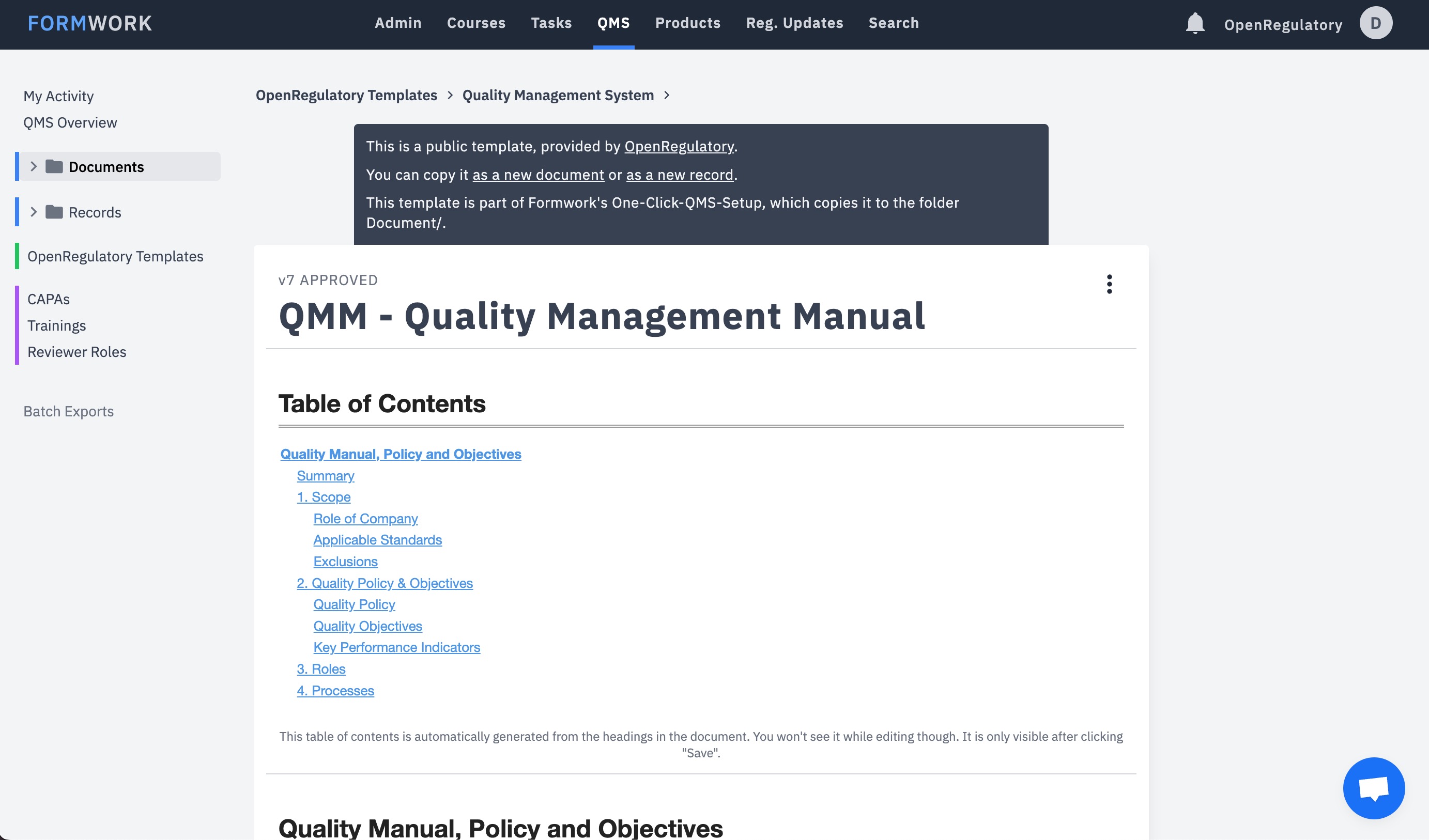
How is medical device regulation developed and how can its usability be improved for start-ups?

Accepted answer
Regulation like the MDR is developed at the EU level as part of the "new legislative framework" for product safety. The process involves EU institutio