When you’re starting out as a medical device startup, the first thing you’ll have to come up with is an intended purpose. And the first thing to worry about is “which risk class is my medical device?”.
The risk classification is a crucial decision because all of your strategic decisions will depend on it. However, the classification is sometimes hard to determine. The framework for the classifications in the EU Medical Device Regulation (MDR) has been set up mainly for hardware medical devices. The lack of software knowledge among the legislative bodies has led to a lot of fuzziness and uncertainty when trying to figure out the classification for a software as a medical device (SaMD).
That’s why the Medical Device Coordination Group coordinated guidance on software classification with the catchy title “MDCG 2019-11”. However, if you open that document, the PDF title actually says “untitled”. Ok, thanks. This document is meant to lift the veil on software MD classification or rather provide a patch to the MDR’s rusty framework. There’s another more recent one to help you with classification called “MDCG 2021-24”. If you want to know what it’s about, please read Oli’s summary. However, that one is just reusing the software classification examples from MDCG 2019-11.
Although there are only 4 risk classes, the MDR makes things a little more complicated by introducing 22 classification rules that address different types of devices. The MDR claims that the classification is a risk-based approach. However, instead of looking at the actual risk of a device, the MDR assumes different device types to have a certain risk. If your device is of a certain type, the MDR assumes a pre-defined risk. This is a confusing detour and makes the whole thing very rigid as there is no space for “innovation”, i.e. classifying things that are not yet existing.
The classification rules can be found in Annex VII of Regulation (EU) 2017/745 on medical devices (MDR). Here certain device types are mapped to the classes I, IIa, IIb or III.
Additionally, class I is further divided into normal class I devices and class Is (sterile), Im (measuring) and Ir (reusable) devices.
While there are 21 rules on all sorts of hardware devices, there’s only one rule for standalone software devices: Rule 11.
Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes is classified as class IIa, except if such decisions have an impact that may cause:
- death or an irreversible deterioration of a person’s state of health, in which case it is in class III; or
- a serious deterioration of a person’s state of health or a surgical intervention, in which case it is classified as class IIb.
Software intended to monitor physiological processes is classified as class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as class IIb.
All other software is classified as class I.
Let me untangle this for you.
There are 3 types of software out there:
- software that provides information which is used to take decisions with certain purposes and impacts
- software intended to monitor physiological processes
- all other software
To get a better feeling for the classes, I’ve compiled a list of examples for you:
Class I
Software Medical Devices:
- Fertility Score Calculator Apps: Mobile apps that use validated algorithms to calculate a fertility score based on basal body temperature and menstruation days.
- IBS App: Symptom reduction of irritable bowel syndrome with lifestyle adjustments
- App for orthopedic issues (e.g. patellar tendinitis): individually adjusted exercise program for home training
- Depression Apps: Psychological exercises and hints for at-home use, no therapy in itself
- Additional support apps: psychological support for accompanying symptoms (e.g., fatigue) of chronic diseases such as multiple sclerosis.
You can find a more comprehensive list here.
Hardware Medical Devices:
- Dental Floss
- Surgical Gloves
- Stethoscopes
- Crutches
Class IIa
Software Medical Devices:
- Ranking Therapy Options: A software that lists and ranks all available chemotherapy options for BRCA-positive individuals
- Finding a cognitive therapy: the software conducts tests with a patient to help the specialist find the right therapy
- Calculating medical values: calculator for risk scores or medication doses informing the therapeutical decision
Hardware Medical Devices:
- Non-invasive MRI scanners
- Infusion pumps for continuous medication delivery
- Blood glucose monitors with alarms
- Electrocardiography (ECG) machines
- Pulse oximeters
- Nebulizers for drug delivery
Class IIb
Software Medical Devices:
- Treatment planning in radiology: calculating radiation dose etc.
- App for depression diagnosis: based on a score resulting from data on patient’s symptoms (e.g. anxiety, sleep patterns, stress etc.).
- Analysis of CT / MRI scans informing diagnosis of serious diseases (e.g. cancer)
- A mobile app intended to analyze a user’s heartbeat, detect abnormalities and inform a physician accordingly (whatever that means)
Hardware Medical Devices:
- Ventilators
- Surgical laser
- Intensive care monitoring equipment
Class III
Software Medical Devices:
- Monitoring software for active implantable devices: e.g. an app monitoring a pacemaker or an insulin pump control software
- Diagnostic image analysis for acute treatment decisions: e.g. in patients with acute stroke
Class III devices are used in immediately life-threatening situations.
Hardware Medical Devices:
- Pacemakers
- Defibrillators
- Implanted prosthetics
- Breast implants
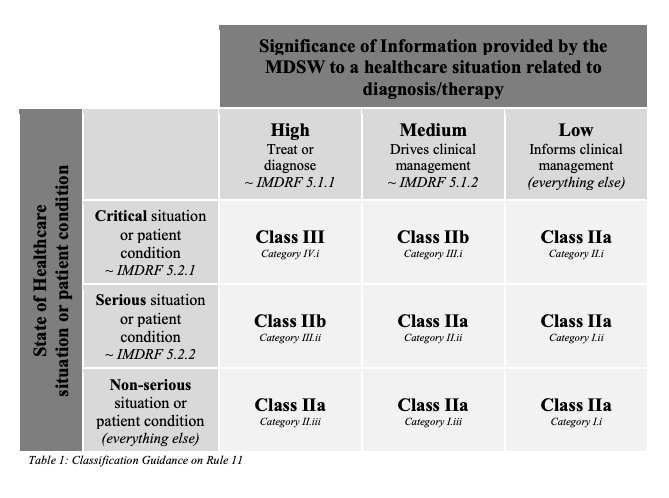
IMDRF Chart
The IMDRF (International Medical Device Regulators Forum) has established a chart to help classify medical devices. It is also referenced in MDCG 2019-11. The table assesses medical devices based on the “significance of information provided […] related to diagnosis/therapy” and the “state of healthcare situation or patient condition”. It makes a lot of sense to view it like this. Unfortunately, this approach neglects the existence of class I software medical devices.
Conclusion
As you can see, software medical devices are by default classified into high-risk classes. It is not easy to understand why Fertility Score Calculators are class I devices while calculating any other score immediately makes the device a class IIa at least.
If you’re suspecting (hoping) that your device could be class I, please read this article.
It is sometimes hard to understand that a MRI scanner has the same risk classification as a software that monitors my blood pressure and analyzes my risk of cardiovascular events.
Let’s make the best of it! Good luck with your classification.
